Oxidative stress is described as cell damage caused by free radicals, or unstable molecules, which can ultimately affect healthy function. The human body creates free radicals to neutralize bacteria and viruses, however, external factors, such as oxygen, pollution, and radiation, can often also produce free radicals. Oxidative stress has been associated with numerous health issues.
Oxidative stress and other stressors turn on internal protective mechanisms which can help regulate the human body's antioxidant response. Nrf2 is a protein which senses levels of oxidative stress and enables the cells to protect themselves from internal and external factors. Nrf2 has also been demonstrated to help regulate genes involved in the production of antioxidant enzymes and stress-response genes. The purpose of the article below is to explain the effects of Nrf2 in cancer.
Abstract
The Keap1-Nrf2 pathway is the major regulator of cytoprotective responses to oxidative and electrophilic stress. Although cell signaling pathways triggered by the transcription factor Nrf2 prevent cancer initiation and progression in normal and premalignant tissues, in fully malignant cells Nrf2 activity provides growth advantage by increasing cancer chemoresistance and enhancing tumor cell growth. In this graphical review, we provide an overview of the Keap1-Nrf2 pathway and its dysregulation in cancer cells. We also briefly summarize the consequences of constitutive Nrf2 activation in cancer cells and how this can be exploited in cancer gene therapy.Keywords: Nrf2, Keap1, Cancer, Antioxidant response element, Gene therapy
Introduction
The Keap1-Nrf2 pathway is the major regulator of cytoprotective responses to endogenous and exogenous stresses caused by reactive oxygen species (ROS) and electrophiles [1]. The key signaling proteins within the pathway are the transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) that binds together with small Maf proteins to the antioxidant response element (ARE) in the regulatory regions of target genes, and Keap1 (Kelch ECH associating protein 1), a repressor protein that binds to Nrf2 and promotes its degradation by the ubiquitin proteasome pathway (Fig. 1). Keap1 is a very cysteine-rich protein, mouse Keap1 having a total of 25 and human 27 cysteine residues, most of which can be modified in vitro by different oxidants and electrophiles [2]. Three of these residues, C151, C273 and C288, have been shown to play a functional role by altering the conformation of Keap1 leading to nuclear translocation of Nrf2 and subsequent target gene expression [3] (Fig. 1). The exact mechanism whereby cysteine modifications in Keap1 lead to Nrf2 activation is not known, but the two prevailing but not mutually exclusive models are (1) the “hinge and latch” model, in which Keap1 modifications in thiol residues residing in the IVR of Keap1 disrupt the interaction with Nrf2 causing a misalignment of the lysine residues within Nrf2 that can no longer be polyubiquitinylated and (2) the model in which thiol modification causes dissociation of Cul3 from Keap1 [3]. In both models, the inducer-modified and Nrf2-bound Keap1 is inactivated and, consequently, newly synthesized Nrf2 proteins bypass Keap1 and translocate into the nucleus, bind to the ARE and drive the expression of Nrf2 target genes such as NAD(P)H quinone oxidoreductase 1 (NQO1), heme oxygenase 1 (HMOX1), glutamate-cysteine ligase (GCL) and glutathione S transferases (GSTs) (Fig. 2). In addition to modifications of Keap1 thiols resulting in Nrf2 target gene induction, proteins such as p21 and p62 can bind to Nrf2 or Keap1 thereby disrupting the interaction between Nrf2 and Keap1 [1], [3] (Fig. 3).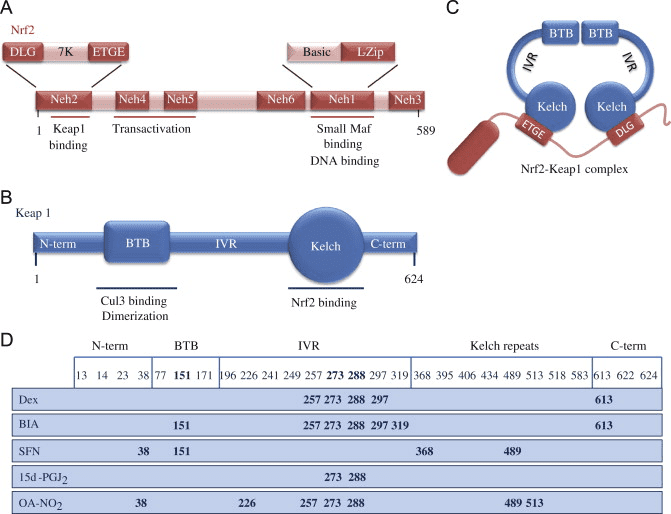
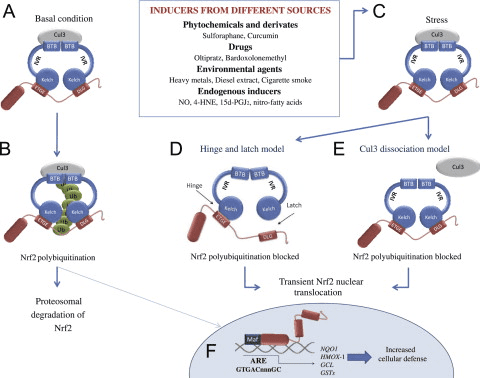
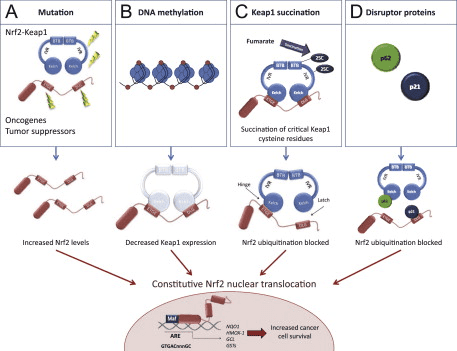
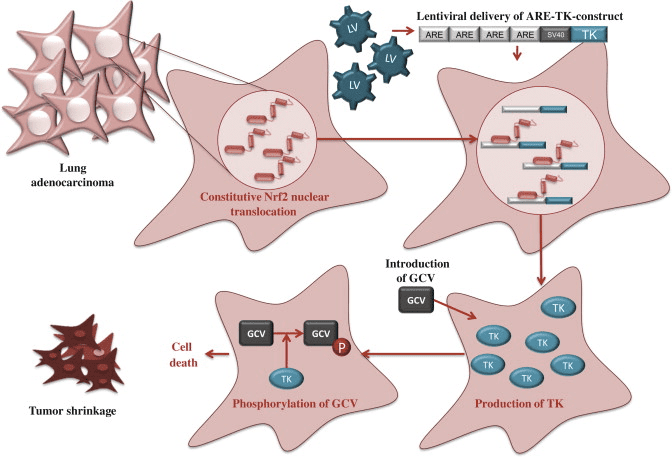
Mechanisms of Activation and Dysregulation of Nrf2 in Cancer
Although cytoprotection provided by Nrf2 activation is important for cancer chemoprevention in normal and premalignant tissues, in fully malignant cells Nrf2 activity provides growth advantage by increasing cancer chemoresistance and enhancing tumor cell growth [4]. Several mechanisms by which Nrf2 signaling pathway is constitutively activated in various cancers have been described: (1) somatic mutations in Keap1 or the Keap1 binding domain of Nrf2 disrupting their interaction; (2) epigenetic silencing of Keap1 expression leading to defective repression of Nrf2; (3) accumulation of disruptor proteins such as p62 leading to dissociation of the Keap1-Nrf2 complex; (4) transcriptional induction of Nrf2 by oncogenic K-Ras, B-Raf and c-Myc; and (5) post-translational modification of Keap1 cysteines by succinylation that occurs in familial papillary renal carcinoma due to the loss of fumarate hydratase enzyme activity [3], [4], [5], [6], [7], [8], [9], [10] (Fig. 3). Constitutively abundant Nrf2 protein causes increased expression of genes involved in drug metabolism thereby increasing the resistance to chemotherapeutic drugs and radiotherapy. In addition, high Nrf2 protein level is associated with poor prognosis in cancer [4]. Overactive Nrf2 also affects cell proliferation by directing glucose and glutamine towards anabolic pathways augmenting purine synthesis and influencing the pentose phosphate pathway to promote cell proliferation [11] (Fig. 4).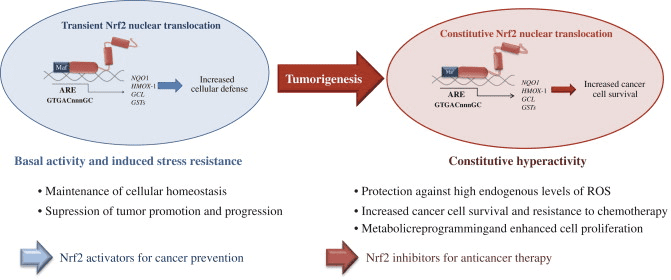

Nrf2 is a master regulator which triggers the production of powerful antioxidants in the human body which help eliminate oxidative stress. Various antioxidant enzymes, such as superoxide dismutase, or SOD, glutathione, and catalase, are also activated through the Nrf2 pathway. Furthermore, certain phytochemicals like turmeric, ashwagandha, bacopa, green tea, and milk thistle, activate Nrf2. Research studies have found that Nrf2 activation can naturally enhance cellular protection and restore balance to the human body.
Dr. Alex Jimenez D.C., C.C.S.T. Insight
Sulforaphane and Its Effects on Cancer, Mortality, Aging, Brain and Behavior, Heart Disease & More
Key sections:
- 00:01:14 - Cancer and mortality
- 00:19:04 - Aging
- 00:26:30 - Brain and behavior
- 00:38:06 - Final recap
- 00:40:27 - Dose
- 00:00:34 - Introduction of sulforaphane, a major focus of the video.
- 00:01:14 - Cruciferous vegetable consumption and reductions in all-cause mortality.
- 00:02:12 - Prostate cancer risk.
- 00:02:23 - Bladder cancer risk.
- 00:02:34 - Lung cancer in smokers risk.
- 00:02:48 - Breast cancer risk.
- 00:03:13 - Hypothetical: what if you already have cancer? (interventional)
- 00:03:35 - Plausible mechanism driving the cancer and mortality associative data.
- 00:04:38 - Sulforaphane and cancer.
- 00:05:32 - Animal evidence showing strong effect of broccoli sprout extract on bladder tumor development in rats.
- 00:06:06 - Effect of direct supplementation of sulforaphane in prostate cancer patients.
- 00:07:09 - Bioaccumulation of isothiocyanate metabolites in actual breast tissue.
- 00:08:32 - Inhibition of breast cancer stem cells.
- 00:08:53 - History lesson: brassicas were established as having health properties even in ancient Rome.
- 00:09:16 - Sulforaphane's ability to enhance carcinogen excretion (benzene, acrolein).
- 00:09:51 - NRF2 as a genetic switch via antioxidant response elements.
- 00:10:10 - How NRF2 activation enhances carcinogen excretion via glutathione-S-conjugates.
- 00:10:34 - Brussels sprouts increase glutathione-S-transferase and reduce DNA damage.
- 00:11:20 - Broccoli sprout drink increases benzene excretion by 61%.
- 00:13:31 - Broccoli sprout homogenate increases antioxidant enzymes in the upper airway.
- 00:15:45 - Cruciferous vegetable consumption and heart disease mortality.
- 00:16:55 - Broccoli sprout powder improves blood lipids and overall heart disease risk in type 2 diabetics.
- 00:19:04 - Beginning of aging section.
- 00:19:21 - Sulforaphane-enriched diet enhances lifespan of beetles from 15 to 30% (in certain conditions).
- 00:20:34 - Importance of low inflammation for longevity.
- 00:22:05 - Cruciferous vegetables and broccoli sprout powder seem to reduce a wide variety of inflammatory markers in humans.
- 00:23:40 - Mid-video recap: cancer, aging sections
- 00:24:14 - Mouse studies suggest sulforaphane might improve adaptive immune function in old age.
- 00:25:18 - Sulforaphane improved hair growth in a mouse model of balding. Picture at 00:26:10.
- 00:26:30 - Beginning of brain and behavior section.
- 00:27:18 - Effect of broccoli sprout extract on autism.
- 00:27:48 - Effect of glucoraphanin on schizophrenia.
- 00:28:17 - Start of depression discussion (plausible mechanism and studies).
- 00:31:21 - Mouse study using 10 different models of stress-induced depression show sulforaphane similarly effective as fluoxetine (prozac).
- 00:32:00 - Study shows direct ingestion of glucoraphanin in mice is similarly effective at preventing depression from social defeat stress model.
- 00:33:01 - Beginning of neurodegeneration section.
- 00:33:30 - Sulforaphane and Alzheimer's disease.
- 00:33:44 - Sulforaphane and Parkinson's disease.
- 00:33:51 - Sulforaphane and Hungtington's disease.
- 00:34:13 - Sulforaphane increases heat shock proteins.
- 00:34:43 - Beginning of traumatic brain injury section.
- 00:35:01 - Sulforaphane injected immediately after TBI improves memory (mouse study).
- 00:35:55 - Sulforaphane and neuronal plasticity.
- 00:36:32 - Sulforaphane improves learning in model of type II diabetes in mice.
- 00:37:19 - Sulforaphane and duchenne muscular dystrophy.
- 00:37:44 - Myostatin inhibition in muscle satellite cells (in vitro).
- 00:38:06 - Late-video recap: mortality and cancer, DNA damage, oxidative stress and inflammation, benzene excretion, cardiovascular disease, type II diabetes, effects on the brain (depression, autism, schizophrenia, neurodegeneration), NRF2 pathway.
- 00:40:27 - Thoughts on figuring out a dose of broccoli sprouts or sulforaphane.
- 00:41:01 - Anecdotes on sprouting at home.
- 00:43:14 - On cooking temperatures and sulforaphane activity.
- 00:43:45 - Gut bacteria conversion of sulforaphane from glucoraphanin.
- 00:44:24 - Supplements work better when combined with active myrosinase from vegetables.
- 00:44:56 - Cooking techniques and cruciferous vegetables.
- 00:46:06 - Isothiocyanates as goitrogens.
Acknowledgments
This work was supported by the Academy of Finland, the Sigrid Juselius Foundation and the Finnish Cancer Organisations.In conclusion, nuclear factor (erythroid-derived 2)-like 2, also known as NFE2L2 or Nrf2, is a protein which increases the production of antioxidants which protect the human body against oxidative stress. As described above, the stimulation of the Nrf2 pathway are being studies for the treatment of diseases caused by oxidative stress, including cancer. The scope of our information is limited to chiropractic and spinal health issues. To discuss the subject matter, please feel free to ask Dr. Jimenez or contact us at 915-850-0900 .
Curated by Dr. Alex Jimenez
Referenced from: Sciencedirect.com

Additional Topic Discussion: Relieving Knee Pain without Surgery
Knee pain is a well-known symptom which can occur due to a variety of knee injuries and/or conditions, including sports injuries. The knee is one of the most complex joints in the human body as it is made-up of the intersection of four bones, four ligaments, various tendons, two menisci, and cartilage. According to the American Academy of Family Physicians, the most common causes of knee pain include patellar subluxation, patellar tendinitis or jumper's knee, and Osgood-Schlatter disease. Although knee pain is most likely to occur in people over 60 years old, knee pain can also occur in children and adolescents. Knee pain can be treated at home following the RICE methods, however, severe knee injuries may require immediate medical attention, including chiropractic care.




