Chiropractor, Dr. Alex Jimenez looks at tendon injury — as well as the ways in which these conditions have been diagnosed And handled…
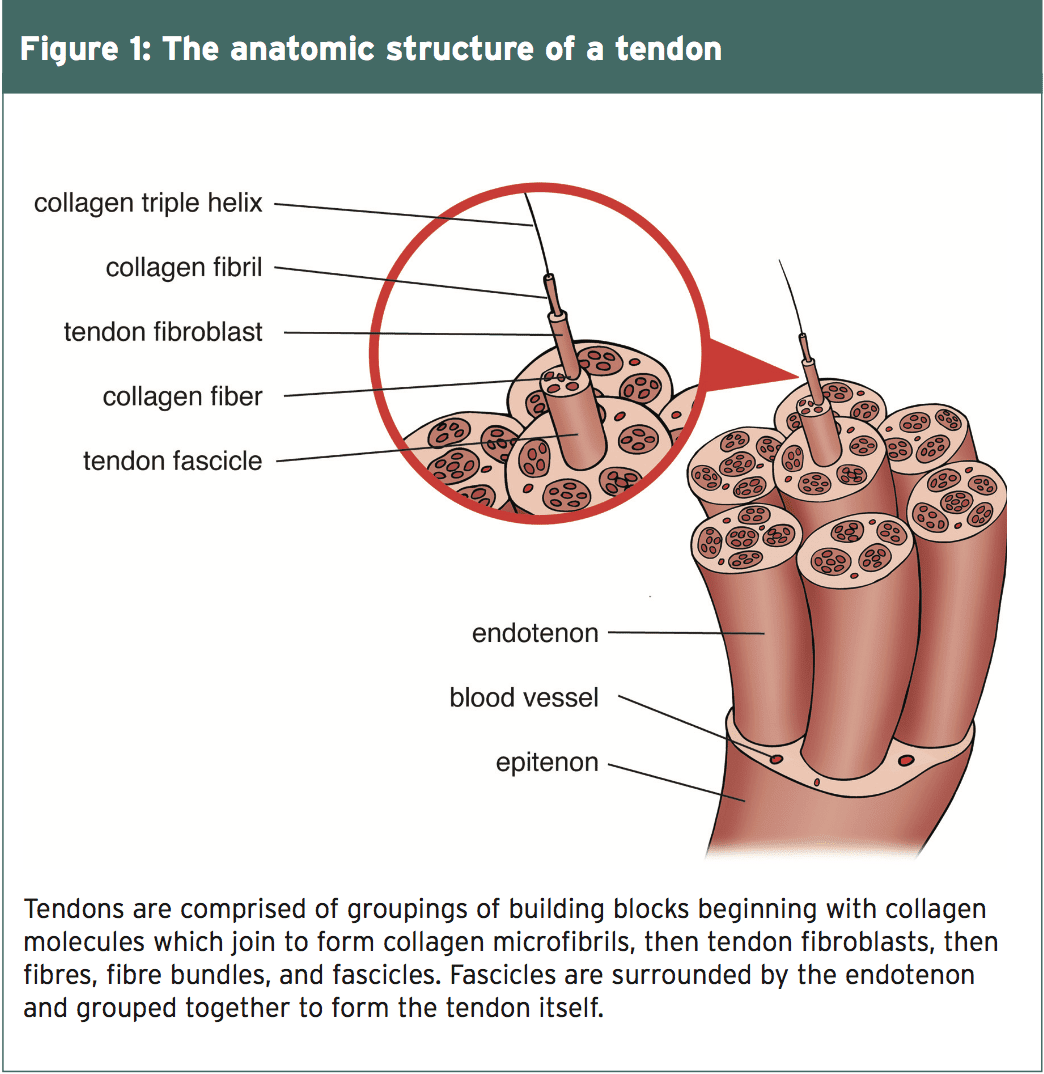
Tendons are bands of tough connective tissue that connect muscles to bones. They conduct the force produced by the muscle to move the bone hence they need to be strong enough to endure the transmission of forces, yet flexible enough to, on occasion, act as pulleys around bony prominences. Collagen, a protein found in the extracellular matrix (ECM) of connective tissue, provides tendons their tensile strength and permits them to be stretched without breaking up. Collagen molecules bind together to form a microfibril, and microfibrils combine one another to make a collagen fibre (see Figure 1). Collagen fibers make a bundle up, and fibre bundles joined together are known as a tendon fascicle.
The fiber tendon fascicles and bundles are sheathed in a thin layer of connective tissue called the endotenon. The endotenon enables the packages and fascicles to move independently of one another, slipping against each other as required to adjust to push and the angle between the bone and the muscle. The endotenon is a continuation of this tissue that encircles the entire tendon, known as the epitenon. Some joints have an additional covering called the peratenon, which, while working with all the tendon, is a arrangement.
The tenocyte, the regulatory mobile within the tendon, modulates the assembly of the collagen inside the tendon and also the secretion of the extracellular matrix. Tenocytes lie in long rows across the collagen fibers and are found in epitenon and the endotenon of this anus. The tenocytes form a connective net of projections that makes it possible for the cells to communicate with each other as the need for degradation or synthesis of collagen fibers. They activate the formation of collagen cells when they experience stresses of short duration. Prolonged tension results in hydration inhibition.
The blood supply to tendons is markedly less than that of the muscle to which they’re attached. The vessels which do exist inside the thoracic are quite modest, and operate together with the fascicles within the endotenon sheath. Some regions of tendon deficiency a blood supply. These areas are particularly vulnerable to degeneration and rupture, as one might suspect.
Tendon Injury
Trainers injure their tendons due to overuse or injury. Of the 32 million musculoskeletal injuries documented in the USA annually, 45 percent of these are injuries to tendons, ligaments, or joint capsules(1). The tendons would be the joints of the rotator cuff of the shoulder, the Achilles tendon, patellar tendon, and elbow extensor tendon.Factors that place a strain on the thoracic and lead to overuse injuries include:
- Abnormal direction of pull due to skeletal misalignment;
- Differences in limb lengths;
- Muscle fatigue or imbalances;
- Hypermobile joints;
- Inflexible muscles;
- Training mistakes;
- Faulty or improperly fitted equipment and sneakers.
Inflammation Plays a Role
Advances in histology allow scientists to now take a closer look at the procedure for tendinopathy. Studies of wounded tendon that is human are hard due to the time a person seeks help the injury is chronic. Animal models are analyzed to reveal acute tendon changes. Researchers from Queen Mary University in London analyzed the response of the tenocytes of horse joints to cyclic loading(two). Fascicle packs from six horses were divided into treatment and control groups. Treatment samples underwent loading strain, while controls remained unloaded.Hours after a cyclic loading Protocol, the collagen cells inside the fascicles of these tendons appeared rounded and unorganised, while the control cells had been long, thin, and aligned along the fascicle. Markers were found in the samples that were treated following their loading cycle, whereas the control samples exhibited few if any markers for inflammation. Researchers reasoned that tendon cells respond especially.
These findings are consistent with other Animal research, which find both an increase in inflammatory markers after episodes of loading or tendon damage, in addition to an increase in the number and size of their tenocytes(2). The proliferation of tenocytes is known to occur in the presence of inflammation; therefore, this reaction is seen as an artefact of a prior inflammatory cascade(3). While degeneration is seen in limb trauma, inflammation might be the instigator of those changes within the tendon during the period of tendon disease.
Further Evidence
Ultrasound examination of symptomatic tendons shows an increase in blood flow to the tendons. Tendons are lacking in blood supply, therefore, to achieve this flow, fresh blood vessels infiltrate the thoracic. This neovascularisation typically occurs in combination with an nerve alongside the blood vessel. The sprouting of new nerves inside the tendon is thought to be the origin of pain in tendinopathy.The influx of blood flow is supposed to be evidence of degeneration in the tendon and an attempt at healing the damaged tissue. However, such neovascularization and neoinnervation could likely not occur without the presence of inflammatory mediators at some Stage(4). Researchers in Cambridge University stage to the fact Look of this tendon body of patients With tendinopathy due to overload or Harm is indistinguishable on ultrasound From those of patients with known Inflammatory diagnoses such as rheumatoid arthritis3.
Biochemical Influences
Cyclooxygenase-2 (COX-2) is an enzyme, which in the presence of nitric oxide stimulates the generation of prostanoids and produces inflammation. Studies indicate that levels of prostanoids are increased in animal tendons exposed to repeated loading(4). In tendons subjected to injected prostanoids, changes found within the tendon are consistent with tendinopathy(3). Therefore, the existence of higher levels of prostanoids in tendons that are diseased is proof of an inflammatory process withinthetendon.Substance P is a peptide secreted cells and by nerves. The presence of substance P in significant quantities in chronic tendinopathy is supposed to be the result of an inflammatory process within the tendon. Substance P causes an increase in the number of tenocytes at a tendon. Therefore, the increased variety of tenocytes observed within an injured limb may be the result of inflammatory mediators such as substance P. Substance P also increases the ratio of collagen III to hydration I molecules in the extracellularmatrix (ECM). At a tendon that is healthy, collagen I is the form found within the ECM. This change in the composition of the collagen at the ECM may well account for the difference in the form and size of the collagen and simultaneous disorganisation observed in the London research(3).
Degeneration Theory
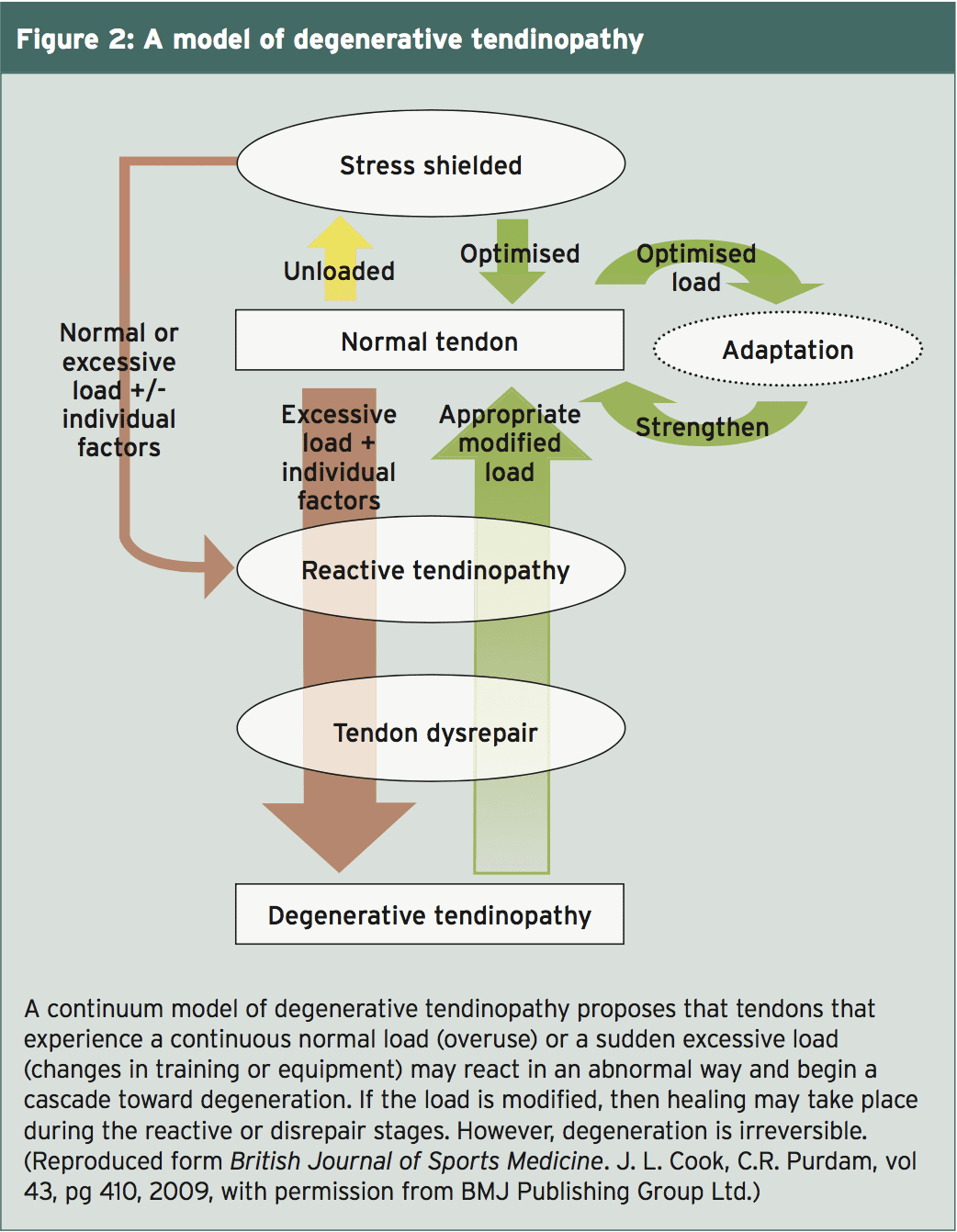
Researchers at Melbourne, Australia, developed a multi-stage version of tendon injury that encircles the current thinking on tendinosis (see Figure 2)(4).) When a tendon undergoes an increased load, it reacts by increasing the production of collagen cells and increasing its endurance to handle the greater force demand. The Australian researchers suggest this short-term proliferative but cell response that is non-inflammatory, is a reactive tendinopathy. Their idea is that the growth in cells is an attempt by the tendon better and therefore, to increase the cross-sectional area handle the load. If the load is diminished, this short-term version is reversible or the tendon has a opportunity to rest until the next stress is put on. A wholesome tendon adapts to the stress. A tendon progresses to stage two and does not recover from the strain.At the next stage, termed thoracic disrepair, the tendon attempts to heal itself by incorporating more cells to the ECM, which raises the protein production of collagen and proteoglycans. They suggest that it’s the increase in the number of proteoglycans that supplies a disorganized appearance to the ECM and affects look and the collagen composition. They note the vascularity at this phase. Their situation is that with modification of the load the composition of the ECM could be altered and healing may take place at this stage.
The final phase is degenerative tendinopathy. The hallmark of the stage is cell death, together with an ECM full of metabolic by-products vessels, and little else and regions of tendon of cells. This stage is deemed irreversible. Degenerative tendinopathy can be found as distinct lesions within a tendon. The wounded limb may display varying phases of degeneration during the tendon.
Two Sides Same Coin
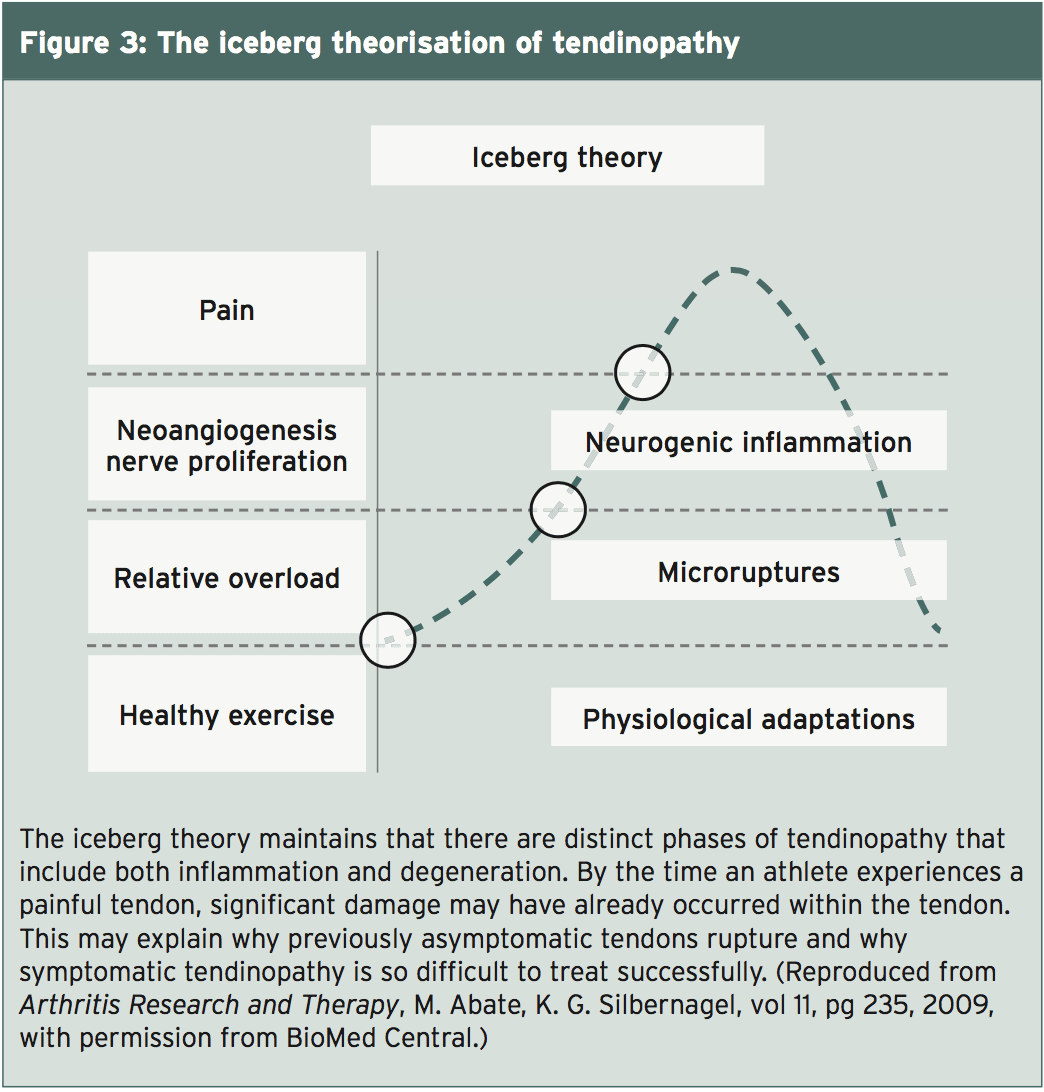
What histology shows about injured tendons, in reality, is the fact that inflammatory changes and degenerative changes are both found in the same tendon(5). A group of scientists from Sweden and Italy suggested a different version for tendinopathy. Termed the “iceberg theory”, this version begins with the premise that regular exercise stimulates the production of new collagen in a tendon (see Figure 3)(5). At exactly the same time, collagen degradation occurs, likely as a way of remodeling the tendon to accommodate the new load(6). Exercise stimulates the production of growth compounds and inflammatory markers, both necessary for stimulation of proliferation and degradation’s wholesome tendon response. In tendons, collagen synthesis wins out in this equation as well as the fascia gets more powerful and larger.When a tendon experiences overload or strain, the collagen fibres within the tendon start to slide past one another, breaking their rectal bonds and inducing a denaturation of the collagen. This micro-trauma is hypothesised to weaken the tendon, and impacts both the ECM and the blood source(5). The temperature inside the thoracic tissue increases. The dissipation of heat is difficult in tendons, due. The scientists theorize that it can be the hyperthermia.
Cessation of remainder, loading, and adequate blood supply are needed to heal from excess strain. Factors are generated that stimulate angiogenesis if the tendon does not have the mandatory blood supply. The look of vessels, which usually contain nerves along with the blood vessels, is considered to weaken the structure of the thoracic(5). The secretion of the two glutamate and substance P from the nerves that are sprouting contributes to tendon cell death in addition to a inflammation. It is that athletes may complain of pain and seek medical attention.
Clinical Correlation
Recognizing that there could be degenerative and inflammatory processes in chronic tendinopathy may improve treatment results. Since inflammation happens early in the course of tendinopathy, non-steroidal anti inflammatory drugs (NSAIDS) and steroid injections might be effective at the onset of pain or when the athlete first notices a ‘tweaking’ or strain of this tendon. Therapy and exercise both function to destroy or reduce the amount of blood vessels and nerves at the gut. In concept, by reducing the amount of vessels that are, the tendon contributes to normal and pain decreases. Eccentric exercise gets the added benefit of stimulating collagen production(4). Therapies, such as soft tissue mobilization, are thought to also stimulate collagen production and also return the ratio of type III collagen to usual to type I collagen within the ECM.New ideas for treatments stimulate. Treatments under analysis include tissue engineering, nitrous oxide scaffolding , exogenous growth factor, platelet rich plasma injection, stem cell injection, and treatment. More study is needed to isolate which joints, at what stage of disease, respond best to which therapy. In the meantime, the recommendation would be to treat any twinge or suspicion of tendinopathy the possibility for healing is greatest and when the anti-inflammatory methods are most effective.
References
1. Birth Defects Res C Embryo Today. 2013 Sept;99(3):203-22
2. Scand J Med Sci Sports. 2014:1-11
3. Br J Sports Med. 2014;48:1553-7
4. Br J Sports Med. 2009;43:409-16
5. Arthritis Res Ther. 2009;11(3):235
6. J Anat. 2008;212:211-28