The benefits or otherwise of hyaluronic acid injections for joint function in athletes remains unclear. Chiropractor, Dr. Alexander Jimenez reviews the science and tries to come up with some best practice recommendations.
Hyaluronic acid (HA) was first discovered in 1934 by Karl Meyer and John Palmer when they isolated a previously unknown substance in the eye of a cow. Further studies uncovered the exact nature and purpose of HA in the human body. HA is composed primarily of sugar and is found in the liquid extracellular matrix of synovial fluid and the vitreous fluid of the eye. Its principal function in the body is to bind water and to lubricate movable parts of the body, such as joints and muscles. Its consistency and tissue-friendliness allows it to be used in skin-care products as an excellent moisturizer.
HA is one of the most hydrophilic (water- loving) molecules in nature and can be described as nature’s moisturizer(1). Intra- articular injections of HA have primarily been advocated for the treatment of symptomatic knee pain in osteoarthritis (OA)(2). The majority of its clinical use however has been to improve pain and function in the symptomatic OA knee; its reported use in the sporting context throughout the literature has been sparse. The purpose of this article therefore is to discuss the basis of HA injections into joints and other tissues for the treatment of pain and improvement of function in the athlete.
What Is HA?
HA is natural-occurring component of synovial joints and is the major constituent of a 1-2-micron layer on the surface of articular cartilage. HA exists naturally in various animal tissues; the highest amounts of HA in the human body are found in the extracellular matrix of soft connective tissues(3). HA is a non-sulphated glycosaminoglycan polysaccharide with a high molecular weight (between 100kDa and 10MDa – see Figure 1)(1). It is generally categorised into three groups based on its molecular weight (MW);- Low MW (less than 1MDa).
- Medium MW (1-2MDa).
- High MW (more than 2MDa).

HA has a short half-life of only three to five minutes in the circulation, which equates to less than a day in tissue, and between one and three weeks in cartilage. This speed of renewal means that this polymer is constantly synthesised and degraded(1).
HA consists of repeating unit of glucuronic acid and N-acetyl-D- glucosamine. The molecular weight is determined by the number of linked repeatingunits(denotedby‘n’–seeFigure 1). Higher molecular weight HA consists of large numbers of linked units.
Mechanism Of Action
The function of HA in the synovial joint is to act on the articular cartilage as a supramolecular aggregate to retain proteoglycans, aggrecan and link protein. It therefore acts as a scaffold for these important extracellular matrix molecules. Loss of this normal HA disrupts cartilage matrix stability and accelerates the destruction of the articular cartilage leading to OA changes in joints(4).
Furthermore, synovial fluid with normal HA concentration acts as a viscous lubricant during slow joint movements, and as an elastic shock absorber during rapid joint movements(5). As well as forming the basis for the proteoglycans of the extracellular matrix of the cartilage, this adaptive ability of the HA in synovial fluid reduces stress and friction on the articular cartilage during slow and fast joint movements(6).
The majority of studies conducted on HA supplementation has been on knees with OA. In osteoarthritic joints, the synovial fluid always contains lower concentrations of HA and shifts towards the lower MW ranges compared to healthy joints with higher than average MW content. This lowering of the MW average leads to a breakdown of the mechanical and viscoelastic properties of the synovial fluid. Furthermore, the lower ranges of MW distributions have been shown to be correlated with increased pain in the OA knee(7-9,10,11).
The synthesis of HA in the OA joint is disrupted by increased levels of pro-inflammatory cytokines, free radicals and proteinases, resulting in an HA with a significantly reduced molecular weight and a reduction in synovial fluid viscoelasticity(6,9,10). When the high MW HA is enzymatically cleaved by the increased levels of the proteinases observed in OA, the lower MW HA no longer can maintain the mechanical integrity of the joint. Moreover, this lower MW HA may be proinflammatory, further accelerating the disease(6). This abnormal HA increases the potential for articular cartilage wear and accelerates progression of the disease.
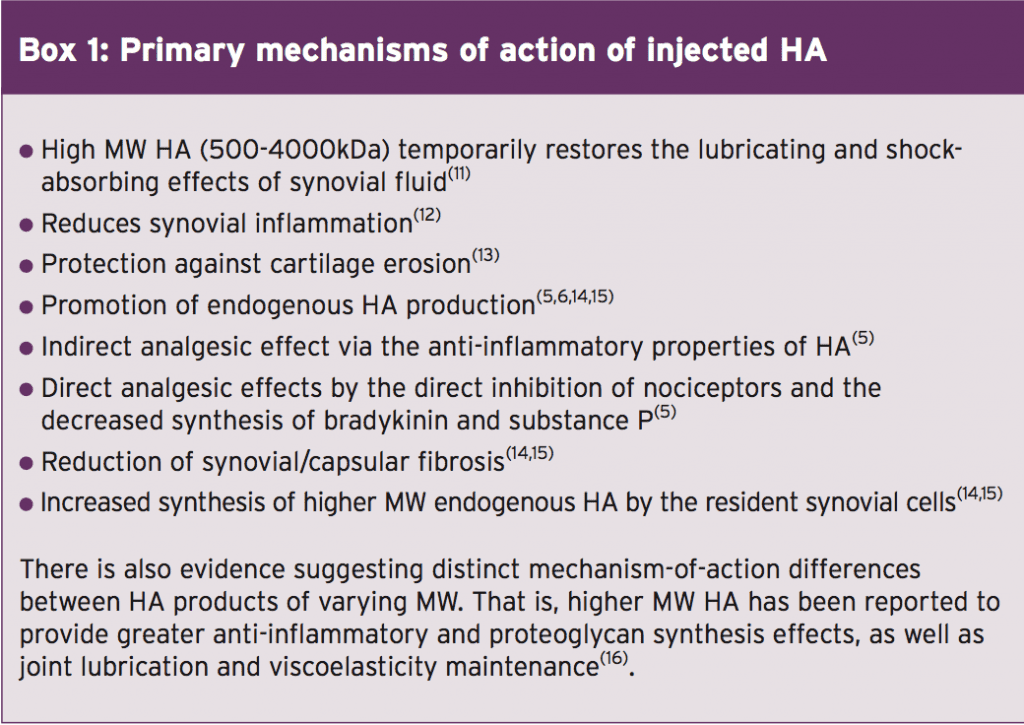
The injection of HA into a joint is thought to restore normal viscoelastic properties of the pathologically altered synovial fluid and improve the average MW distribution within the joint. This procedure is known as ‘viscosupplementation’(9). The primary mechanisms of action of exogenous (injected) HA are summarised in box 1.
The active ingredients are sodium hyaluronate in a concentration of about 20mg per dose. The number of injections per course range from a single injection for Synvisc to five for Hyalgan or Supartz. The total dose of HA per series varies from 45mg for Orthovisc to 125mg for Supartz. Hyalgan, Synvisc, Supartz and Orthovisc are naturally derived products from purified HA extracted from rooster comb. Euflexxa, Durolane, Ostenil and the recently introduced Supartz are made by a different process in which their HA is derived from an engineering-based process that extracts HA from fermented bacterial sources(21). Finally, it has been found that HAs with a MW of less than 500kDa have not been so effective in improving joint function or relieving pain.
Anecdotally, it has been the practice by some sports medicine practitioners to inject hip and knee joints with HA to improve pain and function in athletes. The mechanisms of action and the efficacy for treatment for this would similar to those mentioned above. It would be commonplace if the athlete has a positive response to repeat the injections every three months during the competitive season.
Another possible avenue for HA injection is in cases of tendinopathy in the athlete. Tendinopathy and tenosynovitis disturbs the natural gliding of the tendon. The application of HA can reduce tendon glide resistance(33,34). Several studies on HA have shown the link between the inhibition of fibroblast proliferation and a reduction in type III collagen concentration in the tendon, with a reduction in the formation of adhesions at the tendon healing site(35). By limiting the proliferation of fibroblasts and by stabilising the concentration of type III collagen, HA may reduce the risk of adhesions.
Furthermore, HA appears to inhibit the expression of key inflammatory mediators(5,36). By reducing the expression of proinflammatory mediators, adding exogenous HA reduces the breakdown of endogenous HA and stimulates synovial synthesis of endogenous HA(37). HA has also recently been used as a peri-tendinous injection to treat tendinopathies such as the epicondylitis(38), patellar tendinopathy and insertional Achilles tendinopathy(37).
References
1. Veterinarni Medicina. 2008;53:397-411
2. Pain Med . 2012 June ; 13(6): 740–753
3. Drugs R D. 2011;11(1):13-27. Review
4. Arthritis Res Ther. 2003;5:54-67
5. Sports Med Arthrosc 2006; 14: 155-16
6. Osteoarthritis and Cartilage. 2005;31:216–224
7. Drugs 1994; 47: 536-566
8. J Rheumatol Suppl 1993; 39: 3-9
9. Semin Arthritis Rheum 1991; 20: 219-240
10. Ann Rheum. Dis. 1993;52(11):817–822
11. BMC Musculoskelet Disord 2011; 12: 19
12. J Orthop Res 2000; 18: 604-612
13. Rheumatol. 2008;47:1172-1178
14. Rheumatol Int. 1987;7:113-122
15. Osteoarthritis Cartilage. 2014. 22(1); 121-127
16. Patient Prefer Adherence 2012; 6: 905-910
17. J Rheumatol Dec. 2002;29(12):2611–4
18. Clin Orthop Relat Res Feb. 2004;419:130–7
19. Osteoarthr Cartil. 2006;14(2):154–62
20. Journal of Pain Research 2010:3 51–56
21. Cleve Clin J Med. 2006; 73:897–910
22. Osteoarthritis Cartilage 2011; 19: 611-619
23. Cochrane Database Syst Rev 2006; (2)
24. J Fam Pract 2005; 54: 58-6
25. CMAJ. 2005; 172:1039–43
26. JAMA. 2003; 290:3115–21
27. BioDrugs. 2012;26:257-268
28. Knee. 2001; 8:93–101
29. J Orthop Sci. 2010; 15:51–6
30. Zhonghua Yi Xue Za Zhi (Taipei). 1997; 59:99–106
31. Clinical Medicine Insights: Arthritis and Musculoskeletal Disorders 2014:7 27–32
32. J Hand Surg Am. 2009;34:1276-1281.
33. Clin Biomech (Bristol, Avon). 2006;21:810-815
34. J Hand Surg Am. 1997;22:818-825
35. Febs Letters. 1999;453:283-287
36. J Mater Sci Mater Med. 2014;25:217-227
37. Sports Med Arthrosc Rehabil Ther Technol. 2010; 2: 4.
38. J Orthop Sci. 2014;19(4):603-11.
39. BMC Musculoskelet Disord. 2015;16(1):284
40. BMC Musculoskeletal Disorders (2017) 18:59
The majority of studies conducted on HA supplementation has been on knees with OA. In osteoarthritic joints, the synovial fluid always contains lower concentrations of HA and shifts towards the lower MW ranges compared to healthy joints with higher than average MW content. This lowering of the MW average leads to a breakdown of the mechanical and viscoelastic properties of the synovial fluid. Furthermore, the lower ranges of MW distributions have been shown to be correlated with increased pain in the OA knee(7-9,10,11).
The synthesis of HA in the OA joint is disrupted by increased levels of pro-inflammatory cytokines, free radicals and proteinases, resulting in an HA with a significantly reduced molecular weight and a reduction in synovial fluid viscoelasticity(6,9,10). When the high MW HA is enzymatically cleaved by the increased levels of the proteinases observed in OA, the lower MW HA no longer can maintain the mechanical integrity of the joint. Moreover, this lower MW HA may be proinflammatory, further accelerating the disease(6). This abnormal HA increases the potential for articular cartilage wear and accelerates progression of the disease.
The injection of HA into a joint is thought to restore normal viscoelastic properties of the pathologically altered synovial fluid and improve the average MW distribution within the joint. This procedure is known as ‘viscosupplementation’(9). The primary mechanisms of action of exogenous (injected) HA are summarised in box 1.

How Is Artificial HA Made?
The HA used in intra-articular injections is produced either from harvested rooster combs (avian-derived – (known as AD-HA) or via in vitro bacterial fermentation (known as Bio-HA)(3,17). This artificial HA is produced as a gel and the viscosity of the gel is proportional to the length of the polymer fibres (expressed as its molecular weight or MW). This viscosity will determine how quickly the product degrades after implantation(1). There are differences in the safety of the AD-HA versus the Bio-HA. The AD-HA variation has a greater potential to cause local reactions at the injection site(18, 19). Potential undesirable side-effects include pain, a feeling of heat, haematoma, redness and swelling(20). Avian-derived proteins have been shown to be the cause of injection site flare up, as antibodies to chicken serum protein are present in patients who demonstrated injection-site adverse reaction after being treated with AD-HA(21). HA preparations typically range from 500 to 6000 kDa in molecular weight. The typical preparations available include sodium hyaluronate (Durolane, Hyalgan, Supartz, Ostenil, Ostenil Plus or Euflexxa), Hylan G-F 20 (Synvisc), and highm o l e c u l a r w e i g h t – H y a l u r o n a n (Orthovisc(22). It is important to note that Ostenil Plus contains mannitol, which is a banned substance on the WADA prohibited drugs list; therefore this may not be appropriate for athletes who undergo routine drug testing.The active ingredients are sodium hyaluronate in a concentration of about 20mg per dose. The number of injections per course range from a single injection for Synvisc to five for Hyalgan or Supartz. The total dose of HA per series varies from 45mg for Orthovisc to 125mg for Supartz. Hyalgan, Synvisc, Supartz and Orthovisc are naturally derived products from purified HA extracted from rooster comb. Euflexxa, Durolane, Ostenil and the recently introduced Supartz are made by a different process in which their HA is derived from an engineering-based process that extracts HA from fermented bacterial sources(21). Finally, it has been found that HAs with a MW of less than 500kDa have not been so effective in improving joint function or relieving pain.
Use Of HA In Sport
The elite-level athlete may be more prone to early joint degeneration than the normal population. This is due to abnormal stresses placed upon the weight bearing joints and due to any previous joint injury that may have negatively affected the articular cartilage of the joints, such as previous meniscal tear in the knee and hip labral pathology(32).Anecdotally, it has been the practice by some sports medicine practitioners to inject hip and knee joints with HA to improve pain and function in athletes. The mechanisms of action and the efficacy for treatment for this would similar to those mentioned above. It would be commonplace if the athlete has a positive response to repeat the injections every three months during the competitive season.
Another possible avenue for HA injection is in cases of tendinopathy in the athlete. Tendinopathy and tenosynovitis disturbs the natural gliding of the tendon. The application of HA can reduce tendon glide resistance(33,34). Several studies on HA have shown the link between the inhibition of fibroblast proliferation and a reduction in type III collagen concentration in the tendon, with a reduction in the formation of adhesions at the tendon healing site(35). By limiting the proliferation of fibroblasts and by stabilising the concentration of type III collagen, HA may reduce the risk of adhesions.
Furthermore, HA appears to inhibit the expression of key inflammatory mediators(5,36). By reducing the expression of proinflammatory mediators, adding exogenous HA reduces the breakdown of endogenous HA and stimulates synovial synthesis of endogenous HA(37). HA has also recently been used as a peri-tendinous injection to treat tendinopathies such as the epicondylitis(38), patellar tendinopathy and insertional Achilles tendinopathy(37).
The therapeutic protocol for HA injections targeting tendinopathies is that only 1 or 2 injections alone are recommended(39).However,comparedto other injection therapies for tendons such as protein-rich plasma (PRP) treatments, where a wide variability in the administered product exists, the molecular weight and the concentration of HA are known; high molecular weight HA seems to have a better effect on tendons in the short- term(40).
Finally, the use of mesenchymal stem cells combined with HA has been shown to be beneficial in the treatment of osteochondral injuries in the joint. Park et al (2017) reported a case of a successful outcome using the composite of mesenchymal stem cells and a HA hydrogel for the treatment of large and deep osteochondral defect of the knee(41). The clinical results at one year and at five and a half years postoperatively suggest that this method can be a viable option in restoring large and deep osteochondral defects. This is the first report of a successful treatment of large osteochondral defect of a human joint by application of allogeneic mesenchymal stem cell-based product.

Finally, the use of mesenchymal stem cells combined with HA has been shown to be beneficial in the treatment of osteochondral injuries in the joint. Park et al (2017) reported a case of a successful outcome using the composite of mesenchymal stem cells and a HA hydrogel for the treatment of large and deep osteochondral defect of the knee(41). The clinical results at one year and at five and a half years postoperatively suggest that this method can be a viable option in restoring large and deep osteochondral defects. This is the first report of a successful treatment of large osteochondral defect of a human joint by application of allogeneic mesenchymal stem cell-based product.

In Summary
HA is a high MW molecule that forms the basis of the extracellular joint matrix. It binds water and creates a ‘gel’ that protects the articular cartilage surfaces through both a lubricating effect and a shock absorption effect. It has been shown that joints suffering from degenerative changes have an abnormally lower concentration of high MWHA, and that this loss of healthy HA may promote the acceleration of OA in the joint via a number of mechanisms. HA intra-articular injections have been shown to be effective in both reducing pain and improving function in the OA joint. However, its use in the athlete has been sparsely studied in the literature. But since many athletes have joints that are subject to high compressive and shear forces due to the nature of their high skill activity, early onset arthritis may be more evident in the athlete particularly if they have suffered joint injuries in the past. Despite the lack of empirical studies, we can reasonably surmise that HA injections may help to improve the function and pain response in these joints. The use of HA in tendinopathies appears to be promising, and newer research on HA coupled with mesenchymal stem cells shows some promise in the treatment of osteochondral lesions in the athlete.References
1. Veterinarni Medicina. 2008;53:397-411
2. Pain Med . 2012 June ; 13(6): 740–753
3. Drugs R D. 2011;11(1):13-27. Review
4. Arthritis Res Ther. 2003;5:54-67
5. Sports Med Arthrosc 2006; 14: 155-16
6. Osteoarthritis and Cartilage. 2005;31:216–224
7. Drugs 1994; 47: 536-566
8. J Rheumatol Suppl 1993; 39: 3-9
9. Semin Arthritis Rheum 1991; 20: 219-240
10. Ann Rheum. Dis. 1993;52(11):817–822
11. BMC Musculoskelet Disord 2011; 12: 19
12. J Orthop Res 2000; 18: 604-612
13. Rheumatol. 2008;47:1172-1178
14. Rheumatol Int. 1987;7:113-122
15. Osteoarthritis Cartilage. 2014. 22(1); 121-127
16. Patient Prefer Adherence 2012; 6: 905-910
17. J Rheumatol Dec. 2002;29(12):2611–4
18. Clin Orthop Relat Res Feb. 2004;419:130–7
19. Osteoarthr Cartil. 2006;14(2):154–62
20. Journal of Pain Research 2010:3 51–56
21. Cleve Clin J Med. 2006; 73:897–910
22. Osteoarthritis Cartilage 2011; 19: 611-619
23. Cochrane Database Syst Rev 2006; (2)
24. J Fam Pract 2005; 54: 58-6
25. CMAJ. 2005; 172:1039–43
26. JAMA. 2003; 290:3115–21
27. BioDrugs. 2012;26:257-268
28. Knee. 2001; 8:93–101
29. J Orthop Sci. 2010; 15:51–6
30. Zhonghua Yi Xue Za Zhi (Taipei). 1997; 59:99–106
31. Clinical Medicine Insights: Arthritis and Musculoskeletal Disorders 2014:7 27–32
32. J Hand Surg Am. 2009;34:1276-1281.
33. Clin Biomech (Bristol, Avon). 2006;21:810-815
34. J Hand Surg Am. 1997;22:818-825
35. Febs Letters. 1999;453:283-287
36. J Mater Sci Mater Med. 2014;25:217-227
37. Sports Med Arthrosc Rehabil Ther Technol. 2010; 2: 4.
38. J Orthop Sci. 2014;19(4):603-11.
39. BMC Musculoskelet Disord. 2015;16(1):284
40. BMC Musculoskeletal Disorders (2017) 18:59




